How to Calculate Concentration - ThoughtCo The standard formula is C mV where C is the concentration m is the mass of the solute dissolved and V is the total volume of the solution. PH - log H 3 O.
How to Calculate the Final Concentration of a Solution.
. HCl H2O -- H3O Cl-. Its 00154 molar and we also know that molarity is equal to moles over liters. So the final concentration in molarity of the solution is.
O FIRST Measure pH of 1M HCl Transfer 1mL of 1M from Tube 1. Stretchto cover tube to mix contents Hold down to mix neat. Its one of the easiest units to calculate.
Find the pH of a 00025 M HCl solution. Moles solute per liter of solution not volume of solvent added since the solute takes up some space symbol. All right so lets start with what we know.
How molarity is used to quantify the concentration of solute and calculations related to molarity. If you have a small concentration find the answer in parts per million ppm to make it easier to follow. Concentration is also sometimes shown with a capital C C or M M and a subscript for the solute.
Definitions of solution solute and solvent. In this problem the initial molarity is 300 M the initial volume is 250 mL or 250 x 10 3 L and the final volume is 0175 L. If the substance is an acid its Molarity is equal to its concentration of H.
In order to caluculate the concentration like above it is necessary to know three points of specific gravity or density purity or content and molecular weight. Then transfer 1mL 1 MIX 2 pH 3 pipet hen transfer 1mL 1 pH 2 pipet 1 MIX 2 pH 3 pipet 1 MIX 2 pH. 5 Easy Ways to Calculate the Concentration of a.
1114 gmL x 1000mL x 100ww100 7813 1426molL. HCl is a strong acid which means it ionizes completely in solution according to the equation. Use these known values to calculate the final molarity M2.
This can be calculated by the following equation. Adding Together Two Solutions Find the Hydronium Ion Concentration given the pH Calculate H from pH CHEMISTRY 201. HCl releases one H ion in the solution so its valence factor 1.
And use for Procedures 3 4. The H3O ion is sometimes abbreviated H. We know the concentration of barium hydroxide.
H 2 0050 mole H 2 50 L 0010 M Br 2 0010 M HBr 0 M. All right so we have 00154 as equal to lets make moles X over liters. PH -log H where H stands for the concentration molarity of H3O ion.
Equation pH -log H in order to solve for concentration Hydroxide ion concentration. The molecular weight of HCl 3646 g. Concentrations may be measured using various units with one very useful unit being Molarity.
Moles solute per liter of solution not volume of solvent added since the solute takes up some space symbol. The pH is then calculated using the expression. A conversion is required.
Using the Mass per Volume Equation. PH is determined by the concentration of H which is frequently summarized as H. How to find the concentration Jan 06 2022 In this formula C 1 is the concentration of the starting solution V 1 is the volume of the starting solution C 2 is the concentration of the final solution and V 2 is the volume of the final solution.
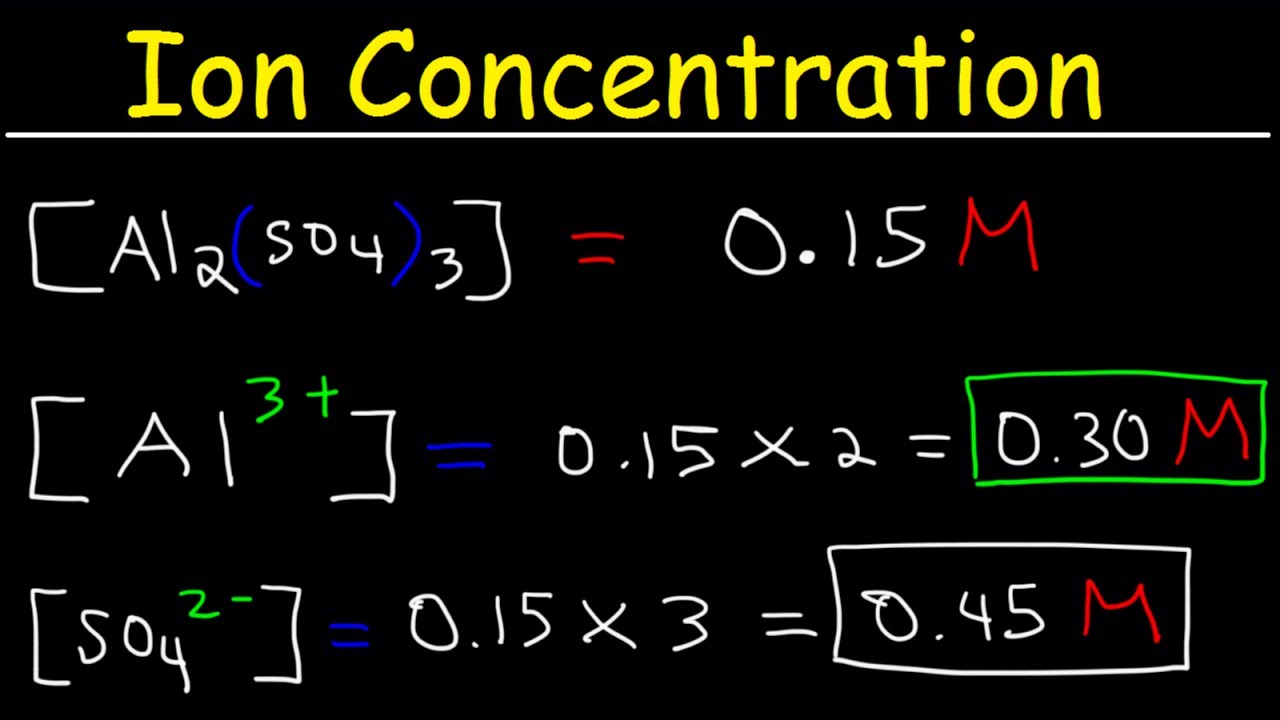
The solute concentration of a solution may be decreased by adding solvent a process referred to as dilution. We will talk more about Molarity in the Titrations page pH pOH 14. Calculate Concentration of Ions in Solution N a2SO4.
The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. PH-log left H right pH logH or pHlog left dfrac 1 left H right right pH logH 1. For example the concentration of hydrogen ion is depicted as H.
429 x 102 M. Concentration in molarity molL or M is often depicted with square brackets around the solute of interest. M M moles liter How to Calculate Concentration - ThoughtCo How to Calculate the Concentration of a Solution.
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter molarity. Solutions - Converting between Percent By Mass and Molarity How to find concentration of H given pH Dilution Problems -. Of the easiest units to calculate.
If the substance is a base its Molarity is equal to its concentration of OH-. In this example they are not. Concentration When Adding Together Two Solutions How to find concentration of H given pH How to Calculate Hydroxide ion OH- Concentration from pH Calculating Concentration of.
So equivalent weight Molecular weight X 36461 3646 g and Number of gram equivalent MassEquivalent weight 053646 00137 So we get the number of gram equivalent 00137. H 2 g Br 2 g 2 HBr g K c 64 700 K Write the equilibrium expression for the reaction. Is expressed in terms of molarity The concentration of ions in a solution depends on dissociation of solute.
Up to 24 cash back OH- 10-pOH - The concentration of OH- is equal to 10 to the power of the negative pOH. By calculating this value by applying this value to the above equation you can know the molar concentration. Adding Together Two Solutions How to find concentration of H given pH How to Calculate Hydroxide ion OH- Concentration from pH Calculating Concentration of Hydronium Ion from a.
Since K c is used in this problem check to see if the given quantities are in moles per liter molarity.
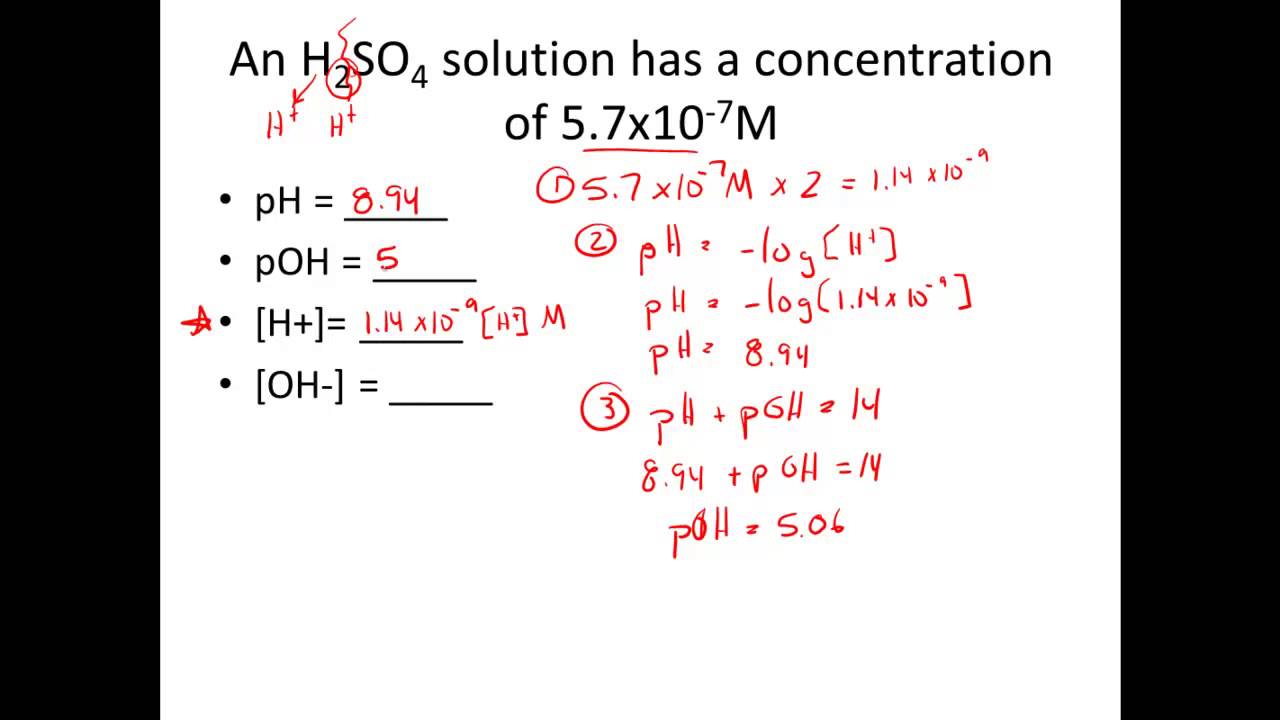
Given H Or Oh Calculate Ph Poh Youtube

Ph Log H Assuming 100 Percent Dissociation If Given Percent Ionization Multiply By The Molarit Chemistry Education Chemistry Lessons Chemistry Classroom

Ion Concentration In Solutions From Molarity Chemistry Practice Problems Youtube


0 Comments